- Categories
-
Soczewka Surgical Gonio (VSGACS)








| The quantity in the package | 1 opak |
| Shipping within | 48 hours |
| Shipping price | The Lack Of |
| The Availability Of |
dostepny
|
| Pełna oferta Volk Optical USA | |
| The bar code | |
| EAN | |
PART #VSGACS
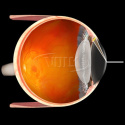
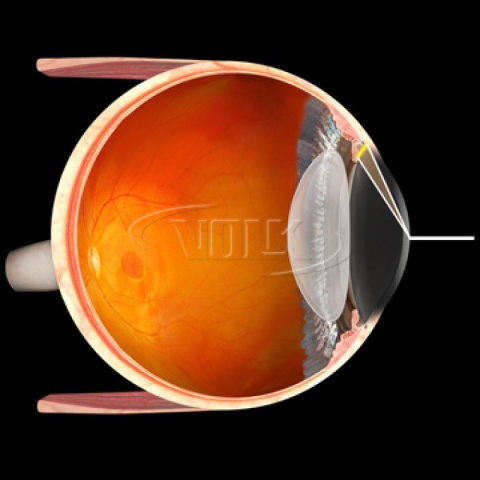
Primary Application - Direct Views for Intraoperative Gonio Procedures
- Lens position can be adjusted relative to the handle: for left hand and right hand or center position
- Applicable for MIGS procedures
- Sterilizable by either steam autoclave or ethylene oxide (ETO)
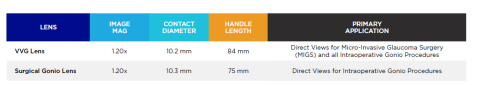
| Image Magnification | Contact Diameter | Ring Diameter | Handle Length |
| 1.20x | 9 mm | 10 mm | 75 mm |


Safety Information
VOLK SURGICAL GONIO ACS LENS:
Do not use a microfiber cloth, as over time these tend to collect dirt and dust which can damage the anti reflective coating on the lens.
Never use a device that shows any sign(s) of damage.
Failure to follow the point-of-use processing steps could adversely affect further decontamination steps.
Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the member state in which the user and/or patient is established.
A thorough, manual cleaning process is recommended. Corrosive cleaning agents (i.e. acids, alkalines, etc) are not recommended. Detergent cleaning agents with neutral ph are recommended.
Do not use detergents that contain any type of emollients.
Wiping the lens with a microfiber cloth will cause lens damage. Be sure to use only a soft, lint-free
cotton cloth.
Ensure the device is completely submerged in the disinfectant solution for the entirety of the recommended or desired soak time. Do not allow the device to become unsubmerged from the disinfectant solution. Exposure to disinfectant solutions beyond the recommended soak time, and/or exposure to higher concentrations of disinfectant solution, will result in accelerated degradation of most Volk product.
Rings may discolor when exposed to Sodium Hypochlorite or Glutaraldehyde. To avoid further degradation please follow only the disinfection procedures indicated for these products in this document. This color change is purely cosmetic and will not affect the function of the lens.
Do not brush lens portion to avoid scratching. Use a soft, cotton cloth.
Carefully examine the device for damage prior to each use. Do not use the device if any surface(s) show(s) any evidence of damage, or patient injury could result.
Contacting surface of the lens element interacts with the cornea, in part, to effect globe support and manipulation. Care should be used to consistently control the amount of the manual force exerted by the surgeon on the patient’s eye with the Alcon AVG lens.
The device ships non-sterile and must be cleaned and sterilized prior to use. See cleaning and sterilization procedures.
Allow the device to cool to room temperature before general or surgical use.
Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the member state in which the user and/or patient is established.
For use by trained personnel only.
Manufacturer details
Volk Optical INC.
7893 Enterprise Dr, Mentor
OH 44060 Mentor,
United States
+1 440-942-6161
volk@volk.com
Responsible person
Rudolf Riester GmbH
Bruckstrasse, 31
72417 Jungingen
Germany
+49747792700
pfister@riester.de
![[{[item.product.name]}]]([{[item.product.photo.url]}] 125w)